
Extraction Methods of Antioxidants from Foods and Medicinal Plants
The exploration and utilization of natural antioxidants derived from foods and medicinal plants have garnered significant attention in recent years due to their potential health benefits and minimal side effects. Extraction, the initial and pivotal step in studying these antioxidants, involves the isolation of bioactive compounds from plant matrices. The efficiency of this process is influenced by multiple factors, including the type and concentration of the extraction solvent, extraction temperature, duration, and pH. Among these, the choice of solvent stands out as a particularly influential factor.
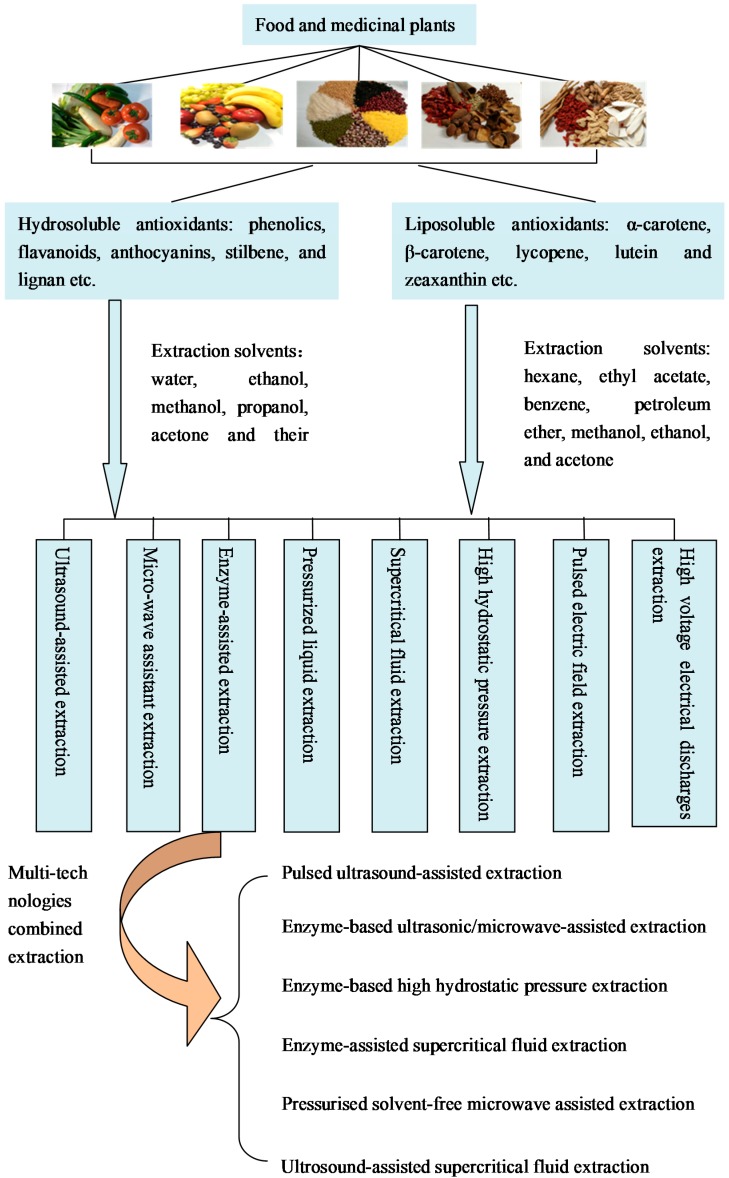
Role of Solvents in Extraction
Solvents play a crucial role in the extraction process as they facilitate the solubilization and subsequent separation of antioxidants from plant materials. The selection of an appropriate solvent is guided by the chemical nature and polarity of the target antioxidant compounds. Antioxidants are broadly classified into two categories based on their solubility: hydrosoluble and lipid-soluble.
Hydrosoluble Antioxidants
Most phenolic compounds, flavonoids, and anthocyanins belong to the hydrosoluble category. These antioxidants are typically extracted using polar or medium-polar solvents. Water, being a polar solvent, is the most natural and eco-friendly choice for extracting hydrosoluble antioxidants. However, pure water extraction may not always be sufficient due to the potential formation of emulsions or the presence of interfering substances. Therefore, aqueous mixtures of organic solvents like ethanol, methanol, propanol, and acetone are commonly used. These solvents enhance the extraction efficiency by increasing the solubility of antioxidants and reducing competition from other plant components.
Ethanol is particularly favored due to its relatively low toxicity, cost-effectiveness, and ability to dissolve a wide range of antioxidants. Methanol, while effective, is less preferred due to its toxicity. Acetone and propanol also find use, often in combination with water, to tailor the extraction conditions to specific antioxidant profiles.
Lipid-Soluble Antioxidants
Carotenoids are examples of lipid-soluble antioxidants that are abundant in many fruits, vegetables, and medicinal plants. These compounds are generally extracted using non-polar or slightly polar solvents. Organic solvents such as hexane, which has a high affinity for lipids, are commonly employed. However, hexane alone may not fully solubilize all carotenoids, necessitating the use of mixtures.
Mixtures of hexane with acetone, ethanol, or methanol have been found to be effective in enhancing the extraction of carotenoids. Similarly, mixtures of ethyl acetate with these organic solvents have also shown promise. The selection of the solvent mixture often depends on the specific carotenoid target and the plant matrix composition.
Other Extraction Factors
While solvents are central to the extraction process, other factors also play important roles:
- Extraction Temperature: Higher temperatures can increase the solubility and diffusion rate of antioxidants, but excessive heat may degrade sensitive compounds.
- Extraction Time: Prolonged extraction times can improve yield but may also lead to degradation or loss of antioxidants.
- Extraction pH: The pH of the extraction medium can affect the ionization state of antioxidants, influencing their solubility and stability.
Advanced Extraction Techniques
In addition to traditional solvent extraction methods, several advanced techniques have been developed to improve the efficiency and selectivity of antioxidant extraction. These include:
- Ultrasound-Assisted Extraction (UAE): Ultrasound waves create cavitation and turbulence in the solvent, enhancing mass transfer and increasing extraction rates.
- Microwave-Assisted Extraction (MAE): Microwave energy rapidly heats the sample, leading to faster solvent penetration and extraction.
- Pressurized Liquid Extraction (PLE): High temperatures and pressures enhance solvent penetration and solubility, allowing for more efficient extraction.
- Supercritical Carbon Dioxide Extraction (SFE-CO2): Carbon dioxide under supercritical conditions acts as a powerful solvent for lipid-soluble antioxidants, offering a non-toxic and environmentally friendly alternative.
The extraction of antioxidants from foods and medicinal plants is a complex process involving multiple variables, with solvents being a key determinant of extraction efficiency. The choice of solvent must be carefully considered based on the polarity and chemical nature of the target antioxidants. By optimizing extraction conditions and exploring advanced techniques, researchers can enhance the yield and purity of antioxidants, paving the way for their broader application in food, pharmaceutical, and cosmetic industries. As the understanding of plant antioxidants deepens, so too will the refinement of extraction methods, ensuring the sustainable and effective utilization of these natural treasures.

